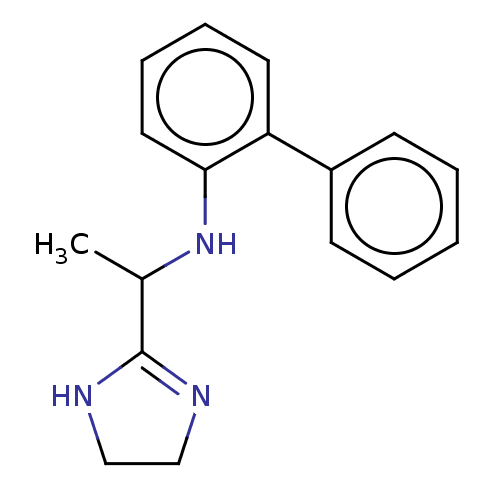
BDBM50473619 CHEMBL130884
SMILES CC(Nc1ccccc1-c1ccccc1)C1=NCCN1
InChI Key InChIKey=QPAIAAWBFFTOPZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50473619
Found 8 hits for monomerid = 50473619
Affinity DataEC50: 3.80E+3nMAssay Description:Agonist activity at human adrenergic alpha-2B receptor expressed in CHO cells assessed as extracellular acidification by cytosensor microphysiometryMore data for this Ligand-Target Pair
Affinity DataEC50: 389nMAssay Description:Agonist activity at human adrenergic alpha-2A receptor expressed in CHO cells assessed as extracellular acidification by cytosensor microphysiometryMore data for this Ligand-Target Pair
Affinity DataEC50: 93nMAssay Description:Agonist activity at human adrenergic alpha-2C receptor expressed in CHO cells assessed as extracellular acidification by cytosensor microphysiometryMore data for this Ligand-Target Pair
Affinity DataKi: 91nMAssay Description:Displacement of [3H]RX821002 from human adrenergic alpha2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(Rat)
University of Camerino
Curated by ChEMBL
University of Camerino
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat cortex membraneMore data for this Ligand-Target Pair
Affinity DataKi: 708nMAssay Description:Displacement of [3H]RX821002 from human adrenergic Alpha-2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.15E+3nMAssay Description:Displacement of [3H]RX821002 from human adrenergic alpha-2B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.26E+4nMAssay Description:Displacement of [3H]idazoxan from imidazoline receptor I-2 binding sites in rabbit kidney membraneMore data for this Ligand-Target Pair