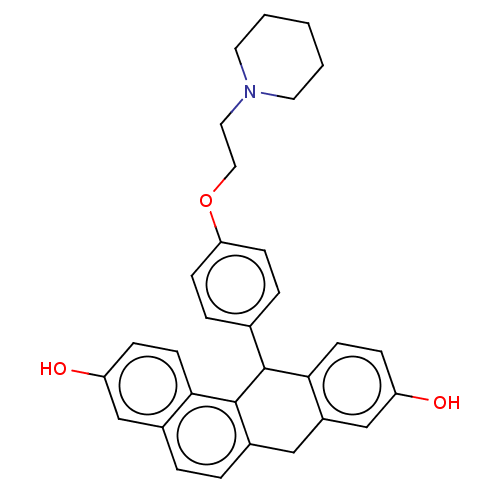
BDBM50474885 CHEMBL360872
SMILES Oc1ccc2C(c3ccc(OCCN4CCCCC4)cc3)c3c(Cc2c1)ccc1cc(O)ccc31
InChI Key InChIKey=FYEARXADVSSUQS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50474885
Found 9 hits for monomerid = 50474885
Affinity DataEC50: 0.5nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair