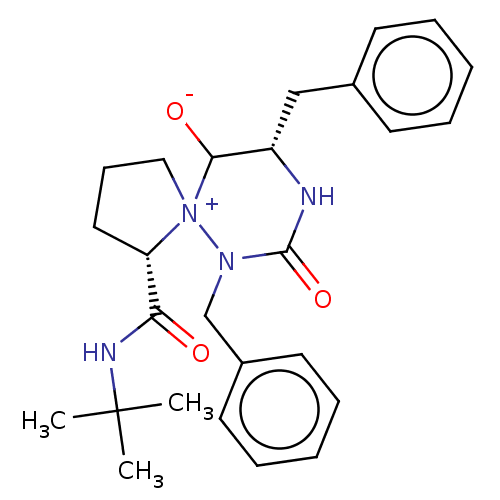
BDBM50480043 CHEMBL466688
SMILES CC(C)(C)NC(=O)[C@@H]1CCC[N+]11C([O-])[C@H](Cc2ccccc2)NC(=O)N1Cc1ccccc1
InChI Key InChIKey=XCJJAAMCALLTFM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50480043
Found 4 hits for monomerid = 50480043
Affinity DataKi: 3.43E+4nMAssay Description:Inhibition of HIV1 protease by Lineweaver-Burke methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.43E+4nMAssay Description:Inhibition of HIV1 protease by Hanes methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.39E+4nMAssay Description:Inhibition of HIV1 protease by Dixon methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.45E+4nMAssay Description:Inhibition of HIV1 protease by FRET assayMore data for this Ligand-Target Pair