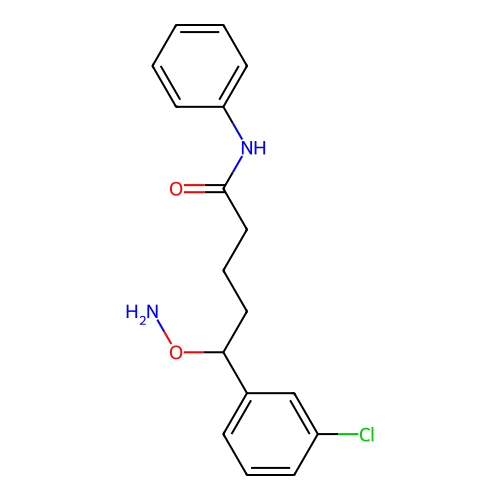
BDBM50507275 CHEMBL4590292
SMILES NOC(CCCC(=O)Nc1ccccc1)c1cccc(Cl)c1
InChI Key InChIKey=MQZXPUVUSFHKGT-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50507275
Found 4 hits for monomerid = 50507275
Affinity DataIC50: 1.23E+5nMAssay Description:Inhibition of human TDO expressed in human T-REx cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.61E+4nMAssay Description:Inhibition of mouse IDO2 expressed in human T-REx cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+3nMAssay Description:Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of IDO1 (unknown origin) using L-tryptophan as substrate by spectrophotometric methodMore data for this Ligand-Target Pair