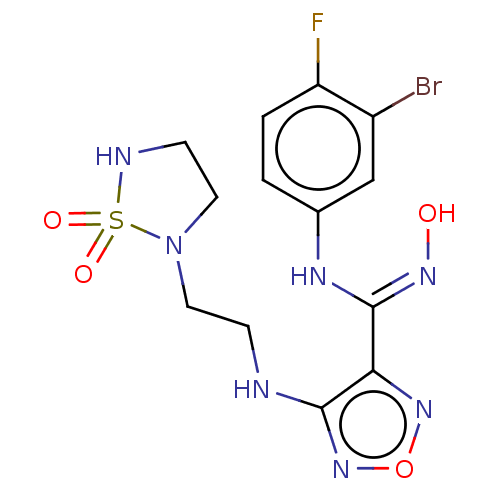
BDBM50513444 CHEMBL4589417
SMILES O\N=C(/Nc1ccc(F)c(Br)c1)c1nonc1NCCN1CCNS1(=O)=O
InChI Key InChIKey=ALBHKCMRPQCXLJ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50513444
Found 3 hits for monomerid = 50513444
TargetIndoleamine 2,3-dioxygenase 1(Human)
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of recombinant human IDO1 expressed in HEK293 cells assessed as N-formylkynurenine formation by measuring kynurenine level incubated for 1...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of recombinant human IDO1 expressed in Escherichia coli BL21 cells using L-tryptophan as substrate incubated for 30 mins by methylene blue...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Shanghai Institute of Materia Medica (Simm)
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair