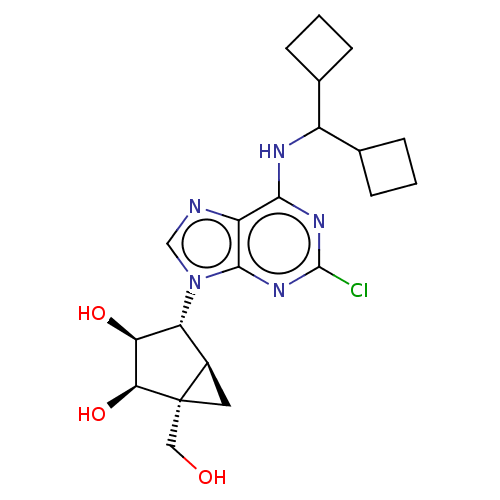
BDBM50517292 CHEMBL4470080
SMILES [H][C@]12C[C@@]1(CO)[C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CCC3)C3CCC3)nc(Cl)nc12
InChI Key InChIKey=KATPAPFGRPJUHJ-UHFFFAOYSA-N
Data 11 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50517292
Found 11 hits for monomerid = 50517292
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Target5-hydroxytryptamine receptor 2B(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 153nMAssay Description:Inhibition of 5HT2B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 238nMAssay Description:Inhibition of 5HT2C (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 612nMAssay Description:Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5-N-methyluronamide from mouse A3A adenosine receptor expressed in CHO cell membranes after ...More data for this Ligand-Target Pair
Affinity DataKi: 2.93E+3nMAssay Description:Inhibition of TSPO (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of muscarinic M3 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.75E+3nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair