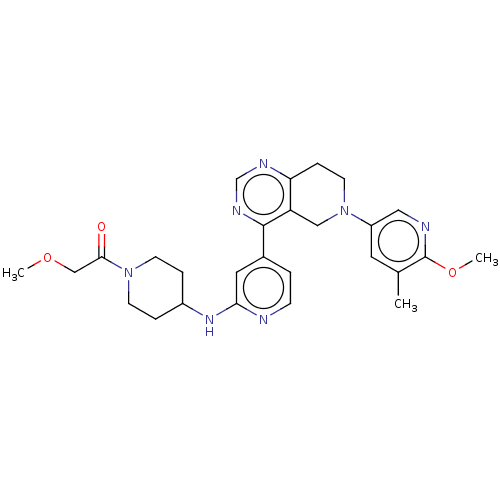
BDBM50517779 CHEMBL4530810
SMILES COCC(=O)N1CCC(CC1)Nc1cc(ccn1)-c1ncnc2CCN(Cc12)c1cnc(OC)c(C)c1
InChI Key InChIKey=RHYXQDJPSRURDD-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50517779
Found 4 hits for monomerid = 50517779
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Human)
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 9.20nMAssay Description:Inhibition of recombinant human PI3Kdelta using PIP2 as substrate after 35 to 40 mins by HTRF assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human PI3Kalpha using phosphatidyl inositol as substrate after 3 hrs by Kinase-Glo Plus reagent-based luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Human)
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human PI3Kgamma using PIP2 as substrate after 35 to 40 mins by HTRF assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Human)
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human PI3Kbeta using PIP2 as substrate after 35 to 40 mins by HTRF assayMore data for this Ligand-Target Pair