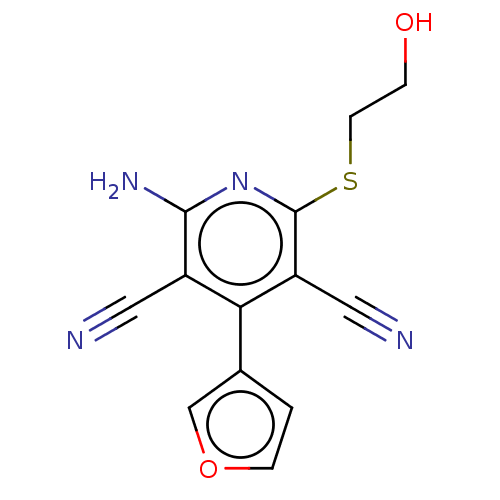
BDBM50528512 CHEMBL4472401
SMILES Nc1nc(SCCO)c(C#N)c(-c2ccoc2)c1C#N
InChI Key InChIKey=KBNKHXPSZAYJCI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50528512
Found 3 hits for monomerid = 50528512
Affinity DataKi: 19nMAssay Description:Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membranes measured after 90 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataEC50: 58nMAssay Description:Inverse agonist activity at human A1AR expressed in CHO cells assessed as increase in forskolin-stimulated cAMP production by alphascreen assayMore data for this Ligand-Target Pair
Affinity DataEC50: 852nMAssay Description:Agonist activity at human A2B expressed in CHO cells assessed as stimulation of cAMP production by alphascreen assayMore data for this Ligand-Target Pair