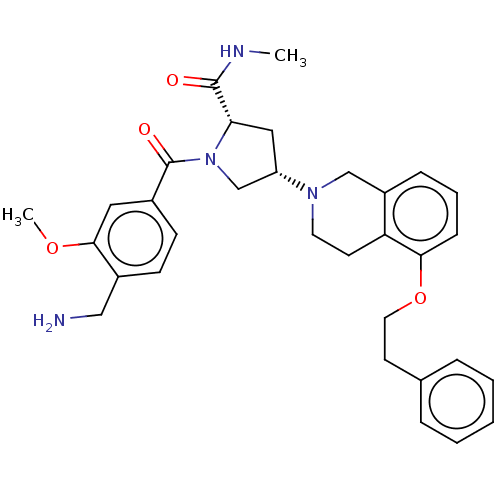
BDBM50533258 CHEMBL4435231
SMILES CNC(=O)[C@@H]1C[C@@H](CN1C(=O)c1ccc(CN)c(OC)c1)N1CCc2c(C1)cccc2OCCc1ccccc1
InChI Key InChIKey=PAZGSUVUHZFUIE-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50533258
Found 4 hits for monomerid = 50533258
Affinity DataIC50: 3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human PRSS1 assessed as enzymatic cleavage of Benzoyl-GlyPro-Arg'Rh110-gammaGlu-OH by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells measured after 90 mins by scintillation proximity assayMore data for this Ligand-Target Pair