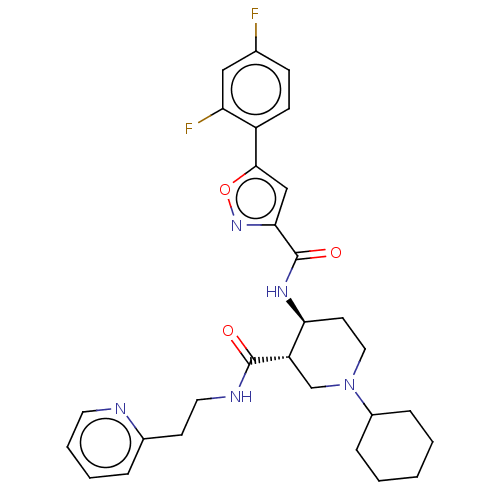
BDBM50555333 CHEMBL4761704
SMILES Fc1ccc(-c2cc(no2)C(=O)N[C@H]2CCN(C[C@@H]2C(=O)NCCc2ccccn2)C2CCCCC2)c(F)c1
InChI Key InChIKey=FUNYICLXDJVARY-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50555333
Found 2 hits for monomerid = 50555333
Affinity DataIC50: 8.70nMAssay Description:Antagonist activity at CXCR7 (unknown origin) expressed in human U2OS cells co-expressing beta-arrestin assessed as reduction in CXCL12-alpha induced...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Idorsia Pharmaceuticals
Curated by ChEMBL
Idorsia Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 960nMAssay Description:Inhibition of human ERG expressed in CHO cells at -40 mV holding potential by Q-patch clamp assayMore data for this Ligand-Target Pair