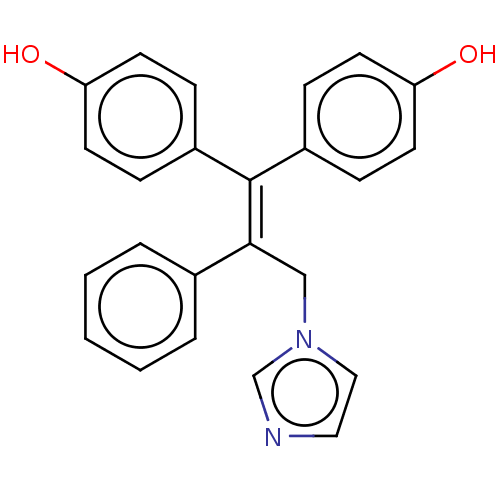
BDBM50558002 CHEMBL4777271
SMILES [#8]-c1ccc(cc1)-[#6](=[#6](\[#6]-n1ccnc1)-c1ccccc1)\c1ccc(-[#8])cc1
InChI Key InChIKey=UHUQIFBURDXQLW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50558002
Found 3 hits for monomerid = 50558002
Affinity DataIC50: 4.80nMAssay Description:Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH...More data for this Ligand-Target Pair
Affinity DataEC50: 27nMAssay Description:Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 41nMAssay Description:Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assayMore data for this Ligand-Target Pair