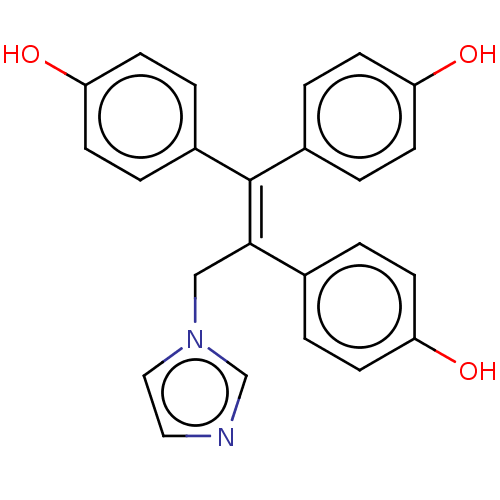
BDBM50558005 CHEMBL4780337
SMILES [#8]-c1ccc(cc1)-[#6](\[#6]-n1ccnc1)=[#6](/c1ccc(-[#8])cc1)-c1ccc(-[#8])cc1
InChI Key InChIKey=HNHMKLMPKIYZTN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50558005
Found 3 hits for monomerid = 50558005
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH...More data for this Ligand-Target Pair
Affinity DataEC50: 74nMAssay Description:Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 98nMAssay Description:Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assayMore data for this Ligand-Target Pair