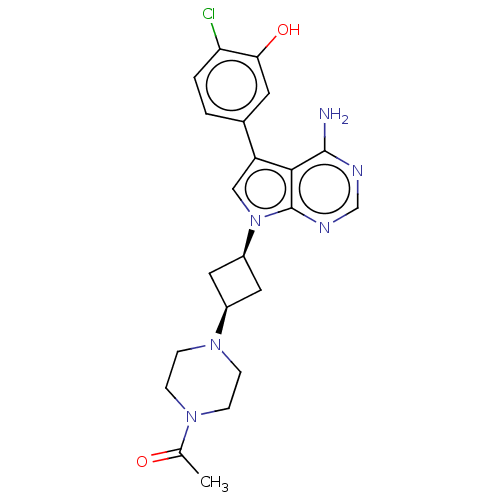
BDBM50575348 CHEMBL4853155
SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2ccc(Cl)c(O)c2)c2c(N)ncnc12
InChI Key InChIKey=HLOASMXRJBZZAG-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50575348
Found 3 hits for monomerid = 50575348
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibition of RET (658 to 1072) (unknown origin) by HTRF assayMore data for this Ligand-Target Pair
TargetKinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret(Human)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Human)
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
The Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 760nMAssay Description:Inhibition of Tel fused KDR (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hrs by cell p...More data for this Ligand-Target Pair