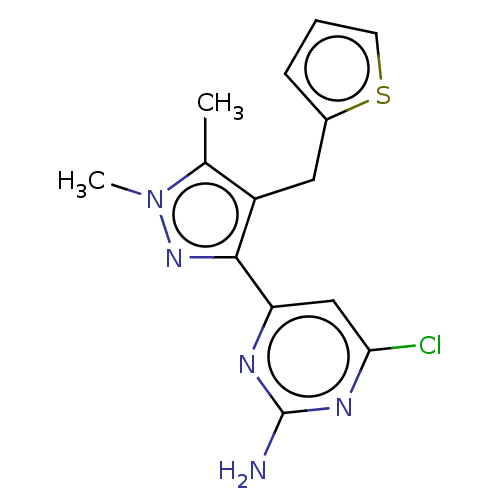
BDBM50577241 CHEMBL4848035::US20240239774, Example 2
SMILES Cc1c(Cc2cccs2)c(nn1C)-c1cc(Cl)nc(N)n1
InChI Key InChIKey=JQJMCWHQDZMPPU-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50577241
Found 3 hits for monomerid = 50577241
Affinity DataIC50: 50nMAssay Description:Assays for sAC activity using purified protein were performed in 100 ul reactions containing 4 mM MgCl2, 2 mM CaCl2, 1 mM ATP, 40 mM NaHCO3, 50 mM Tr...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair