BDBM50583626 CHEMBL5077503
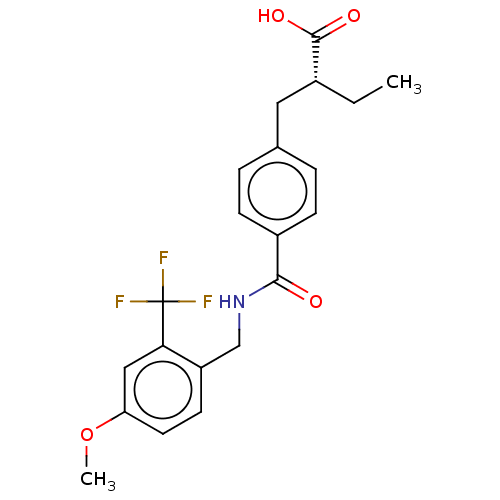
SMILES CC[C@H](Cc1ccc(cc1)C(=O)NCc2ccc(cc2C(F)(F)F)OC)C(=O)O
InChI Key InChIKey=VPAHYLNIDWFBPW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50583626
Found 3 hits for monomerid = 50583626
Affinity DataEC50: 270nMAssay Description:Agonist activity at recombinant N-terminal His6 -tagged GFP fused PPARgamma LBD (unknown origin) (203 to 477 residues) expressed in Escherichia cell ...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of recombinant human C-terminal sEH (222 to 555 residues) expressed in Escherichia coli BL21-DE3 using PHOME as substrate assessed as redu...More data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Agonist activity at human GAL4 DBD-fused PPARgamma LBD (203 to 505 residues) transfected in HEK293T cells assessed as fold activation incubated for 1...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)