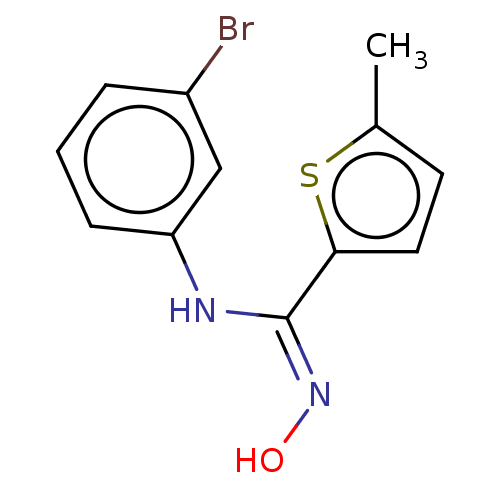
BDBM50584076 CHEMBL5083059
SMILES Cc1ccc(s1)C(\Nc1cccc(Br)c1)=N\O
InChI Key InChIKey=IXDWQKVBIPDMHS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50584076
Found 3 hits for monomerid = 50584076
TargetIndoleamine 2,3-dioxygenase 1(Human)
Taiwan National Health Research Institutes
Curated by ChEMBL
Taiwan National Health Research Institutes
Curated by ChEMBL
Affinity DataEC50: 54nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Taiwan National Health Research Institutes
Curated by ChEMBL
Taiwan National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base...More data for this Ligand-Target Pair
Affinity DataIC50: 5.83E+3nMAssay Description:Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr...More data for this Ligand-Target Pair