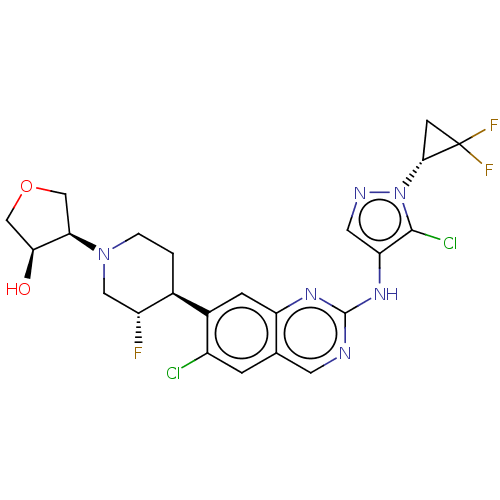
BDBM50584332 CHEMBL5076397
SMILES c1c2cnc(nc2cc(c1Cl)[C@@H]3CCN(C[C@H]3F)[C@@H]4COC[C@@H]4O)Nc5cnn(c5Cl)[C@@H]6CC6(F)F
InChI Key InChIKey=JCYBYWFFRFKLHB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50584332
Found 4 hits for monomerid = 50584332
Affinity DataIC50: 0.600nMAssay Description:Inhibition of recombinant N-terminal GST-fused LRRK2 G2109S mutant (970 to 2527 residues) (unknown origin) preincubated with enzyme for 15 mins follo...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of ERK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of tachykinin NK1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.70E+3nMAssay Description:Inhibition of human ERG by voltage clamp assayMore data for this Ligand-Target Pair