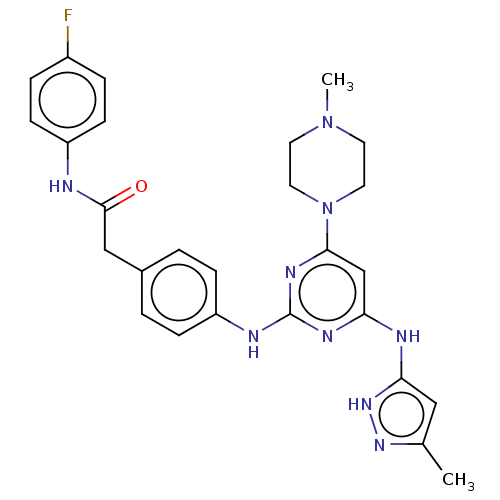
BDBM50585631 CHEMBL5078090
SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Nc2ccc(CC(=O)Nc3ccc(F)cc3)cc2)n1
InChI Key InChIKey=LDBYMVVKYRHKMX-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50585631
Found 2 hits for monomerid = 50585631
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
University of Arkansas For Medical Sciences
Curated by ChEMBL
University of Arkansas For Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.40nMAssay Description:Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Human)
University of Arkansas For Medical Sciences
Curated by ChEMBL
University of Arkansas For Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human RET V804M mutant using 5-FAM- peptide as substrate preincubated for 1 hr followed by substrate addition in presence of ATP by Lin...More data for this Ligand-Target Pair