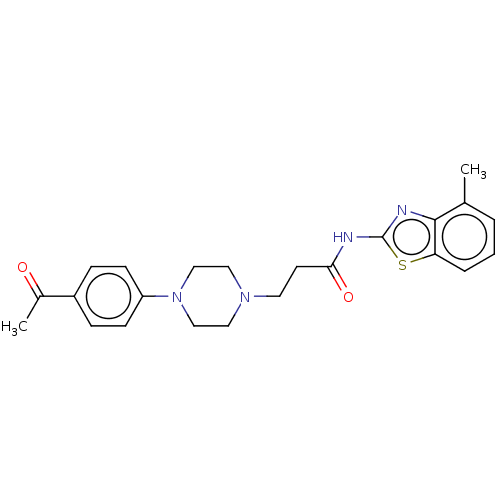
BDBM50593152 CHEMBL5176580
SMILES CC(=O)c1ccc(cc1)N1CCN(CCC(=O)Nc2nc3c(C)cccc3s2)CC1
InChI Key InChIKey=LVMJSGWUSMSUOD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50593152
Found 5 hits for monomerid = 50593152
Affinity DataKi: 187nMAssay Description:Displacement of [3H]ifenprodil from recombinant human GluN2B NMDA receptor (unknown origin) expressed in dexamethasone-induced mouse L-M(TK-) cell af...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]DTG from sigma 2 receptor in rat liver membranes measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain membrane measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.04E+3nMAssay Description:Inhibition of human recombinant BuChe using butyrylthiocholine iodide as substrate incubated for 10 mins by DTNB reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.82E+3nMAssay Description:Inhibition of human recombinant AChe using acetylthiocholine iodide as substrate incubated for 10 mins by DTNB reagent based Ellman's methodMore data for this Ligand-Target Pair