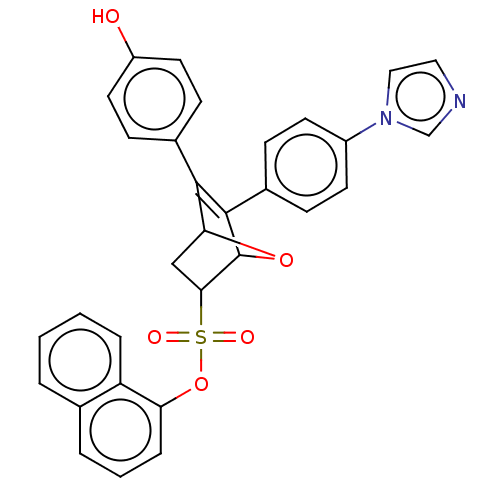
BDBM50618353 CHEMBL5411799
SMILES Oc1ccc(cc1)C1=C(C2OC1CC2S(=O)(=O)Oc1cccc2ccccc12)c1ccc(cc1)-n1ccnc1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50618353
Found 3 hits for monomerid = 50618353
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human recombinant aromatase using ARO as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Binding affinity to ERalpha (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 6.05E+3nMAssay Description:Binding affinity to ERbeta (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair