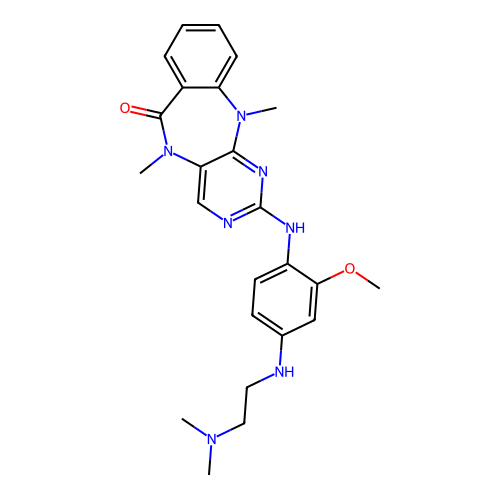
BDBM50652940 CHEMBL5686992
SMILES COc1cc(NCCN(C)C)ccc1Nc1ncc2c(n1)N(C)c1ccccc1C(=O)N2C
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50652940
Found 3 hits for monomerid = 50652940
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of LRRK2 G2019S mutant (unknown origin) using LRRKtide as substrate incubated for 1 hrs in presence of ATP by Adapta TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of N-terminal 6His-tagged human ERK5 expressed in baculovirus infected Sf21 cells co-expressing HA-tagged human MEK5-DD using ARKKRRHPSGPP...More data for this Ligand-Target Pair
Affinity DataEC50: 240nMAssay Description:Inhibition of EGF-stimulated ERK5 autophosphorylation in human HeLa cells preincubated with compound for 1 hrs followed by EGF stimulation for 17 min...More data for this Ligand-Target Pair