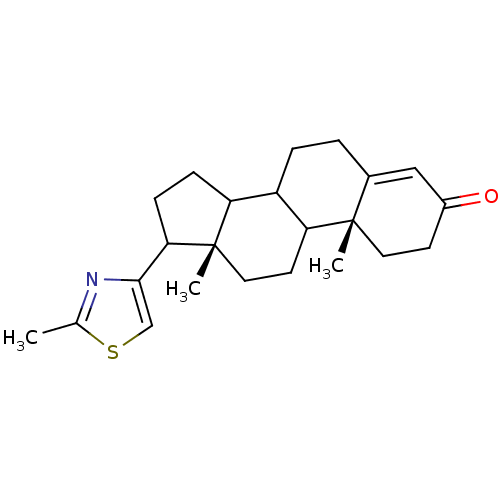
BDBM54382 (10R,13S)-10,13-dimethyl-17-(2-methyl-1,3-thiazol-4-yl)-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one::(10R,13S)-10,13-dimethyl-17-(2-methyl-4-thiazolyl)-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one::(10R,13S)-10,13-dimethyl-17-(2-methylthiazol-4-yl)-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one::MLS000737176::SMR000528404::cid_16682413
SMILES Cc1nc(cs1)C1CCC2C3CCC4=CC(=O)CC[C@]4(C)C3CC[C@]12C
InChI Key InChIKey=NIYPNKHDGWDAOX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 54382
Found 3 hits for monomerid = 54382
TargetNucleotide-binding oligomerization domain-containing protein 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 2.24E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.42E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 2(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.56E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair