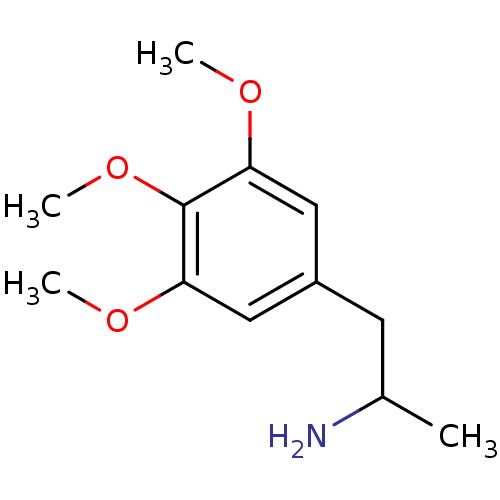
BDBM50005256 (+/-)1-Methyl-2-(3,4,5-trimethoxy-phenyl)-ethylamine::1-Methyl-2-(3,4,5-trimethoxy-phenyl)-ethylamine::CHEMBL30336::TMA
SMILES COc1cc(CC(C)N)cc(OC)c1OC
InChI Key InChIKey=WGTASENVNYJZBK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 55 hits for monomerid = 50005256
Found 55 hits for monomerid = 50005256
Affinity DataEC50: 41.3nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataEC50: 47.4nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Human)
University of Bath
Curated by PDSP Ki Database
University of Bath
Curated by PDSP Ki Database
TargetNicotinic acetylcholine receptor(Cape York rat)
R. J. Reynolds Tobacco
Curated by PDSP Ki Database
R. J. Reynolds Tobacco
Curated by PDSP Ki Database
Affinity DataKi: 477nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 537nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 537nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 749nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 1.68E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 2.07E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKd: 2.51E+3nMAssay Description:Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundusMore data for this Ligand-Target Pair
Affinity DataKi: 2.86E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.04E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.37E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 4.60E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 5.01E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 5.71E+3nMAssay Description:Compound was tested for binding affinity towards 5-HT1C (5-HT1C) receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Human)
University of Bath
Curated by PDSP Ki Database
University of Bath
Curated by PDSP Ki Database
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair