BDBM41536 US8865706, 15
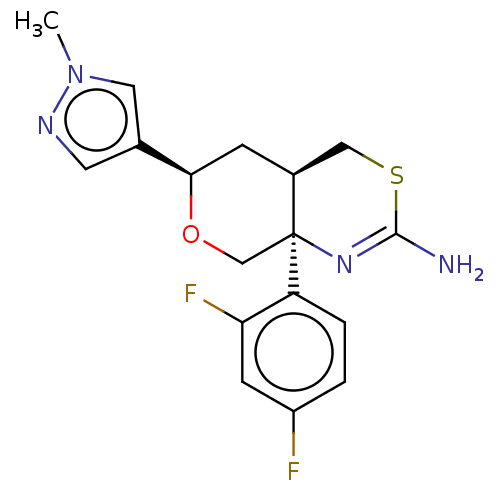
SMILES Cn1cc(cn1)[C@H]2C[C@H]3CSC(=N[C@]3(CO2)c4ccc(cc4F)F)N
InChI Key InChIKey=JYDYMWJEZLKIBS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 41536
Found 10 hits for monomerid = 41536
Affinity DataIC50: 4nMAssay Description:Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f...More data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Diseas...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of human ERG expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+4nMAssay Description:Inhibition of CatD (unknown origin) assessed as fluorescence polarization by cell free assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)