BDBM50459086 CHEMBL4217428
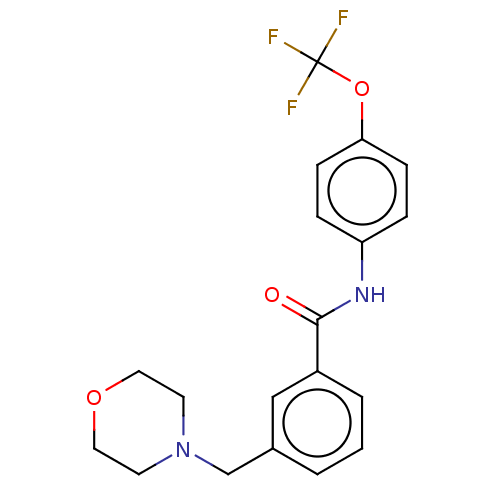
SMILES c1cc(cc(c1)C(=O)Nc2ccc(cc2)OC(F)(F)F)CN3CCOCC3
InChI Key InChIKey=MYWULUKAXYAFSH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50459086
Found 3 hits for monomerid = 50459086
TargetTyrosine-protein kinase ABL1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKd: 2.00E+3nMAssay Description:Binding affinity to ABL1 (64 to 515 residues) (unknown origin) expressed in Escherichia coli by NMR analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Displacement of [3H]dofetilide from human ERG by high throughput assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)