BDBM50505544 CHEMBL4550510
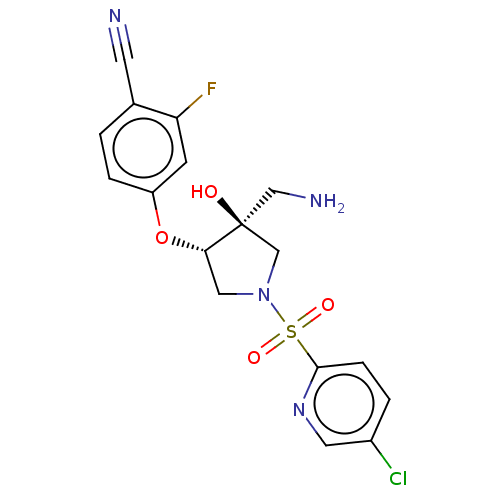
SMILES c1cc(c(cc1O[C@H]2CN(C[C@]2(CN)O)S(=O)(=O)c3ccc(cn3)Cl)F)C#N
InChI Key InChIKey=IQEGRQYYSXOUJI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50505544
Found 3 hits for monomerid = 50505544
TargetTransient receptor potential cation channel subfamily V member 4(Human)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Antagonist activity at BacMam virus expressing human TRPV4 transduced in BHK/AC9 or HEK MSR2 cells assessed as inhibition of GSK634775-induced Ca2+ f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human ERG by electrophysiologyMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)