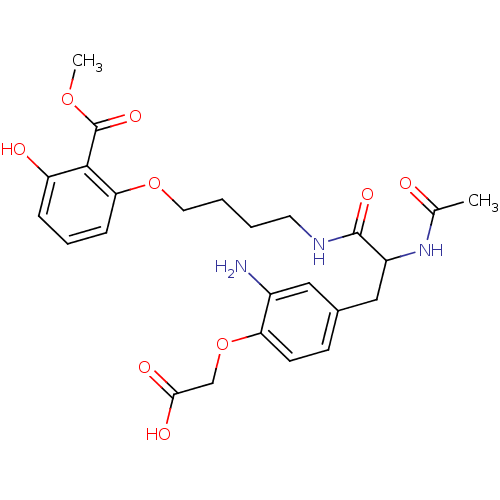
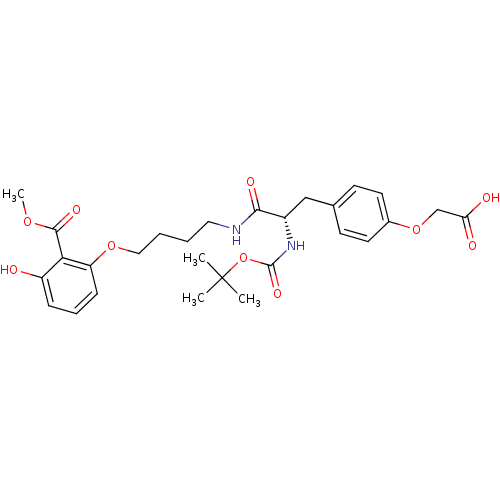
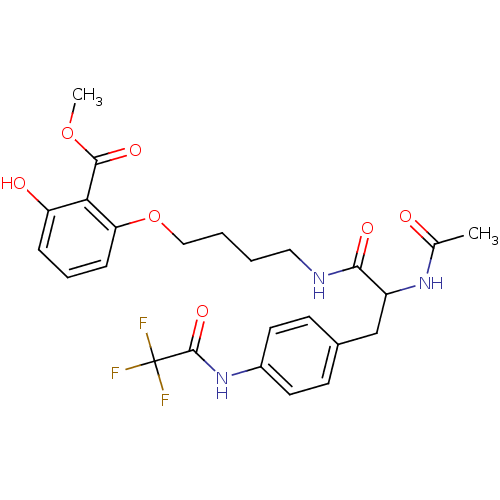
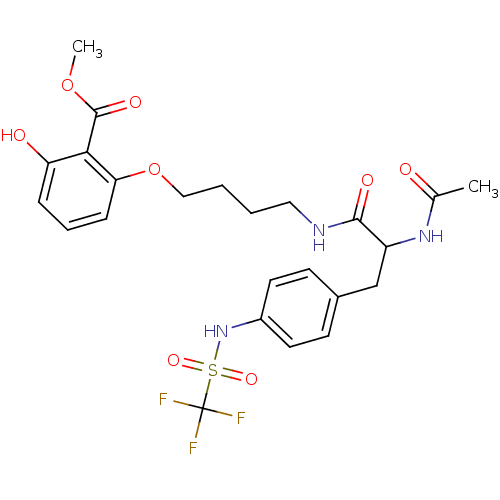
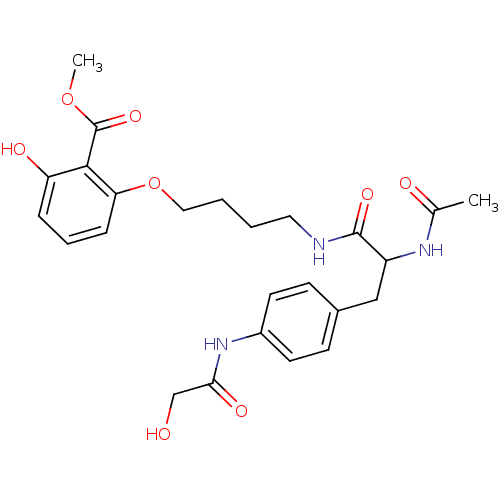
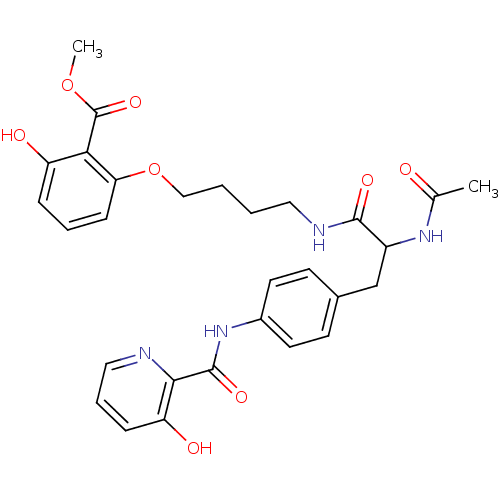
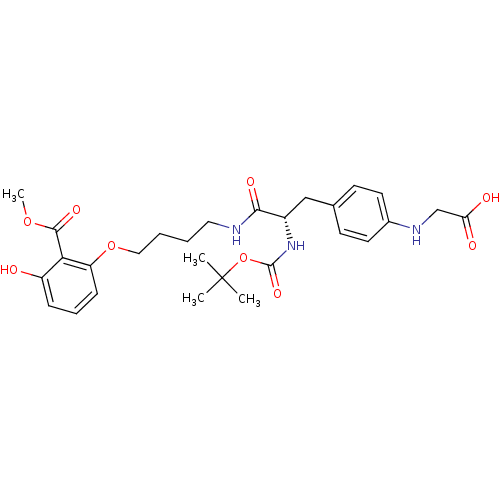
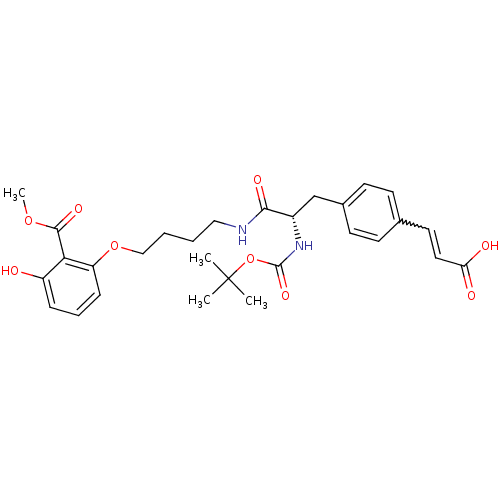
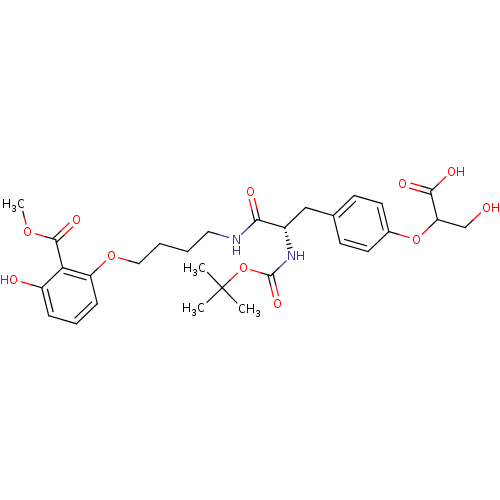
Report error Found 18 Enz. Inhib. hit(s) with all data for entry = 1764
Affinity DataKi: 2.20E+3nM ΔG°: -32.0kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 6.10E+3nM ΔG°: -29.5kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 8.80E+3nM ΔG°: -28.6kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+3nM ΔG°: -28.5kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nM ΔG°: -26.8kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 6.50E+4nM ΔG°: -23.7kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.41E+5nM ΔG°: -21.8kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+5nM ΔG°: -21.1kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+5nM ΔG°: -20.7kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
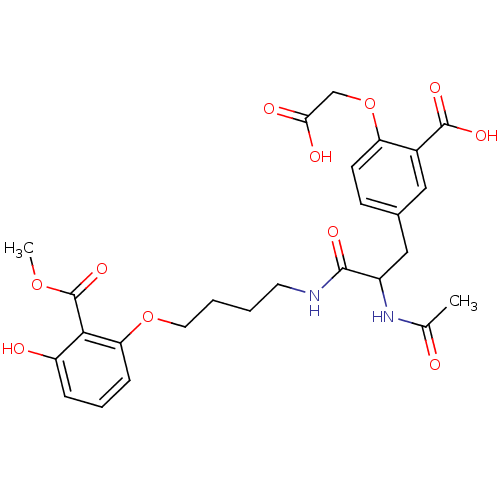
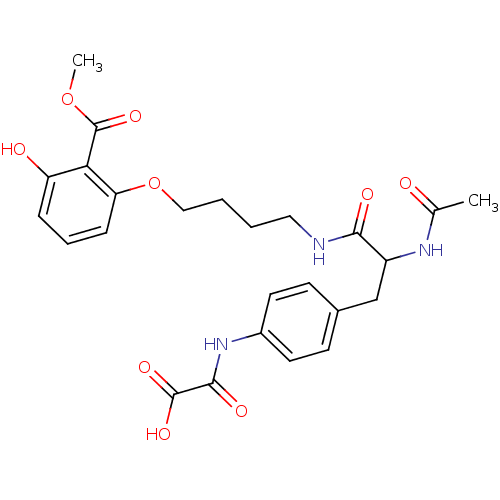
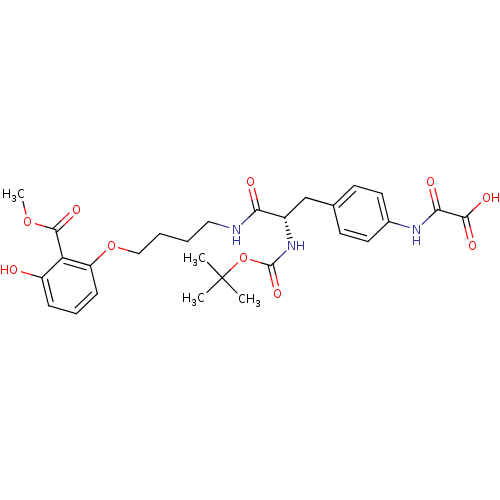
 3D Structure (crystal)
3D Structure (crystal)