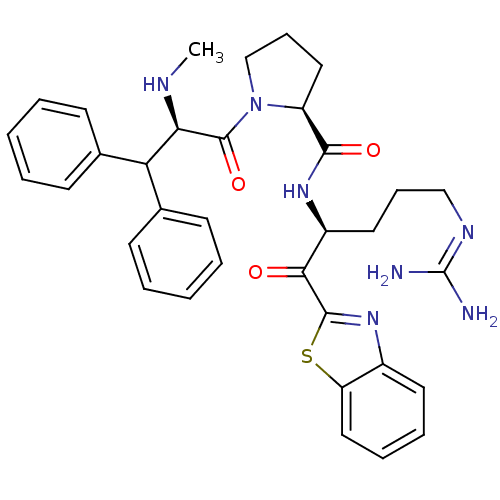
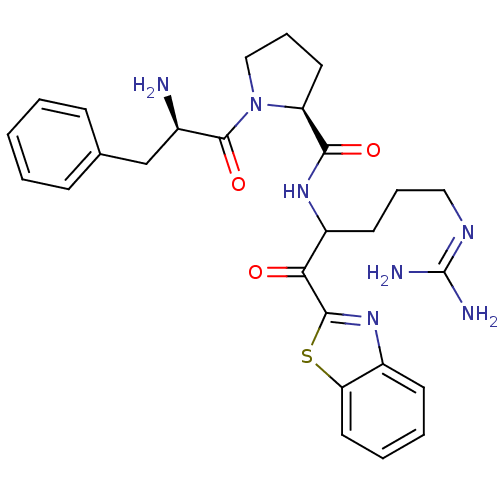
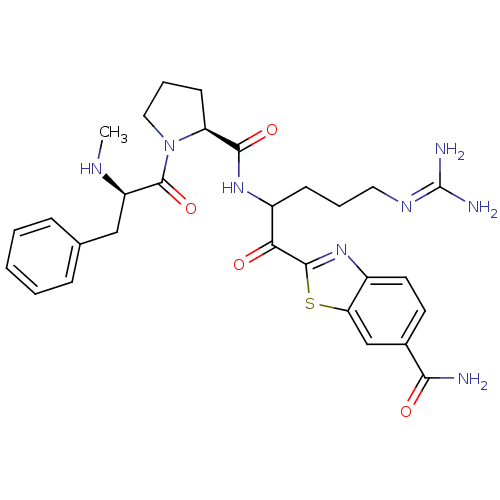
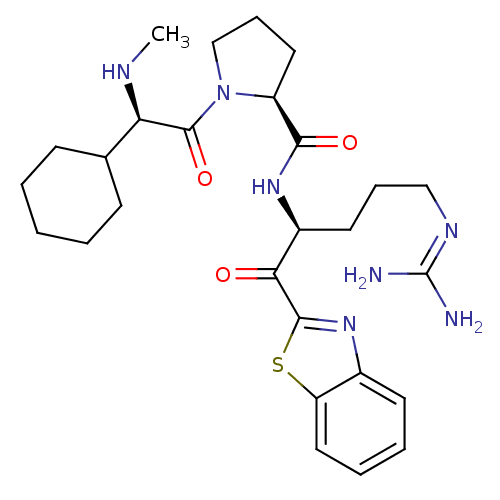
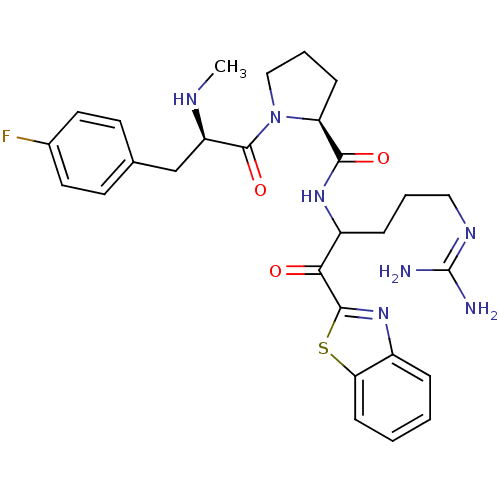
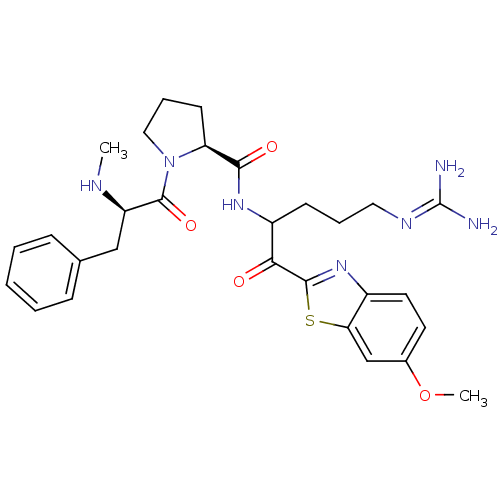
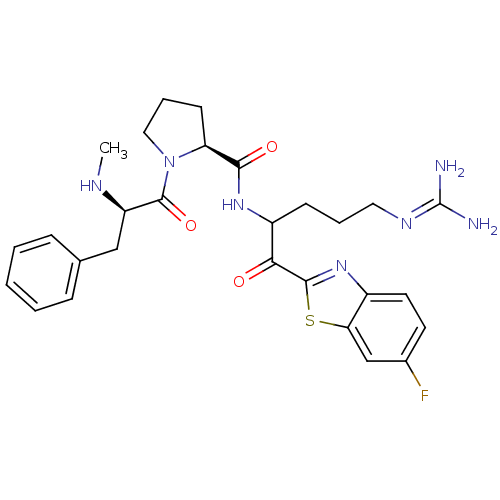
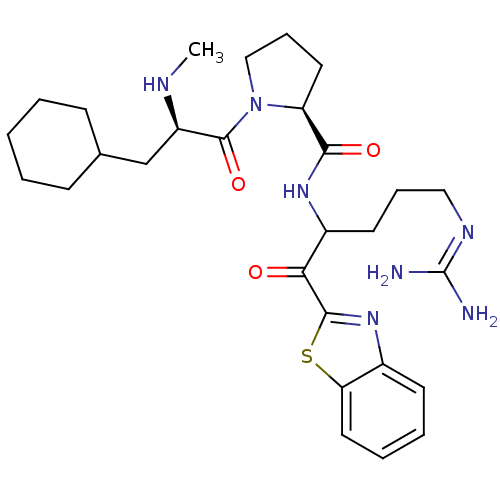
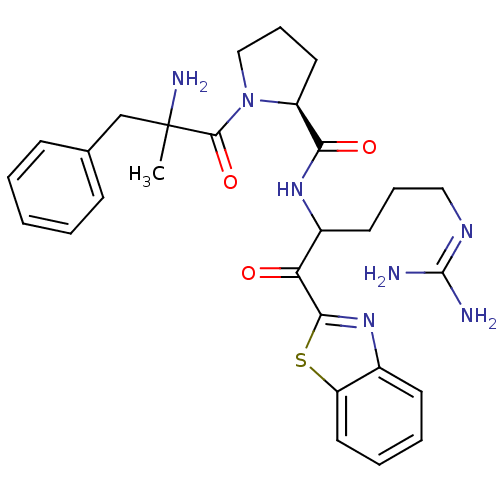
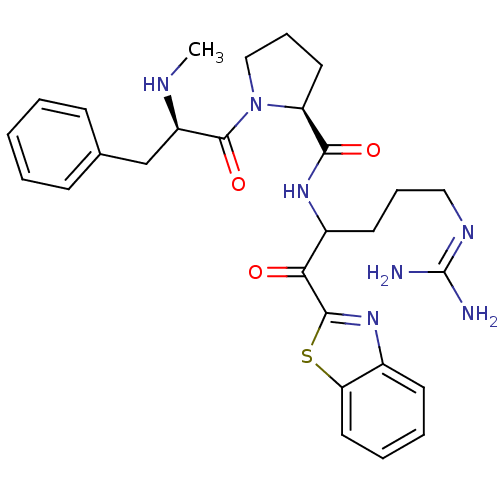
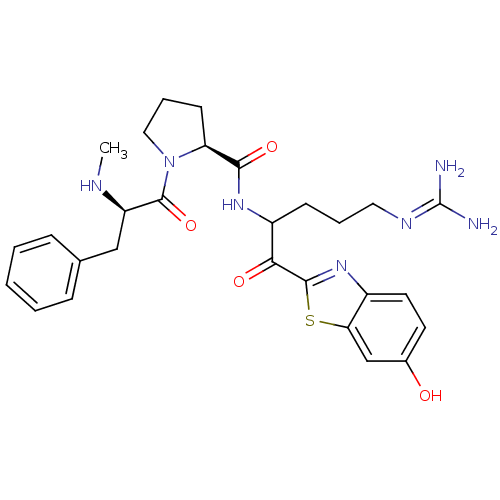
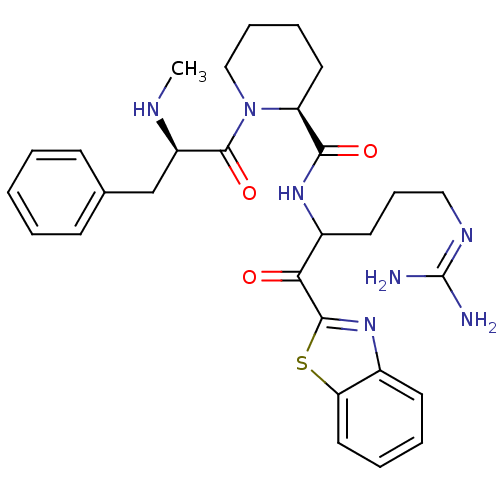
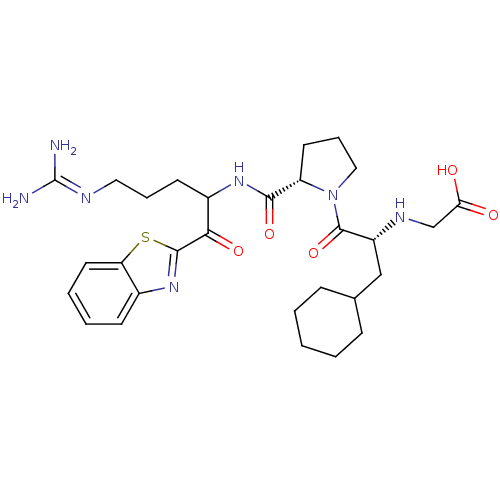
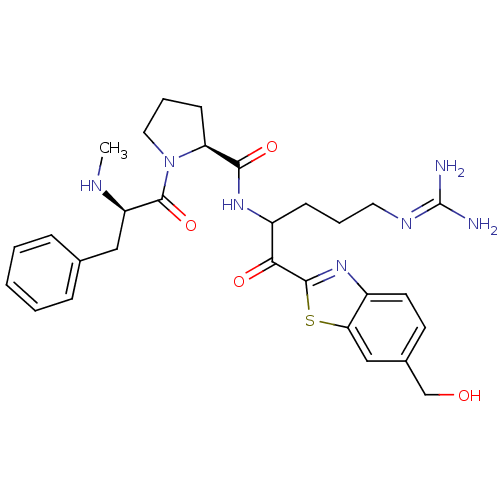
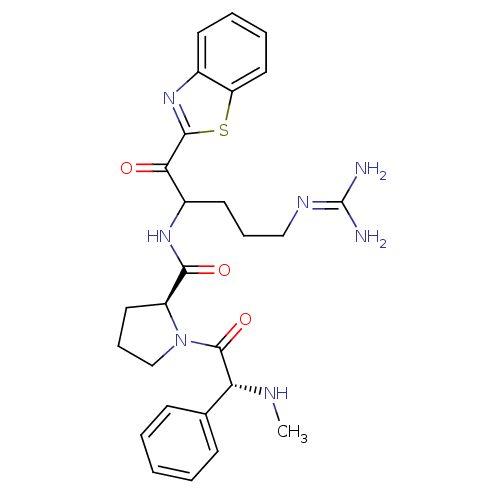
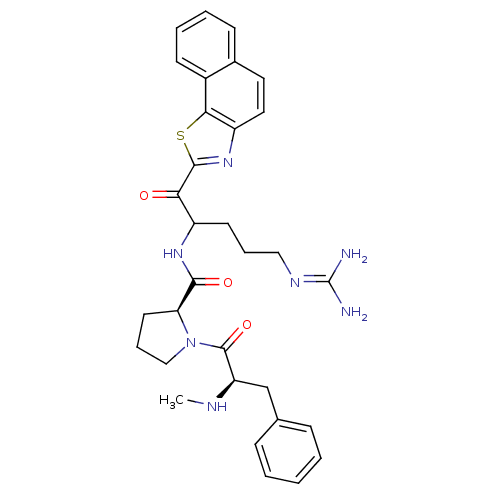
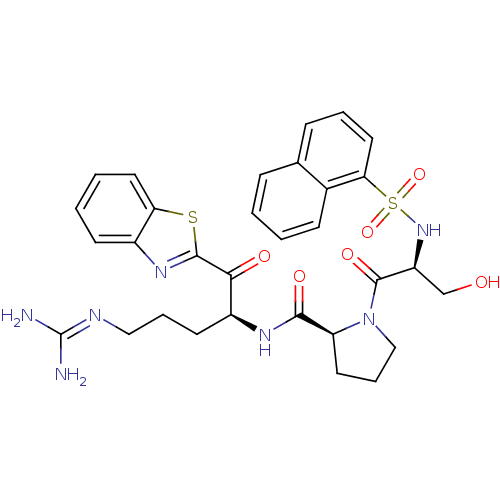
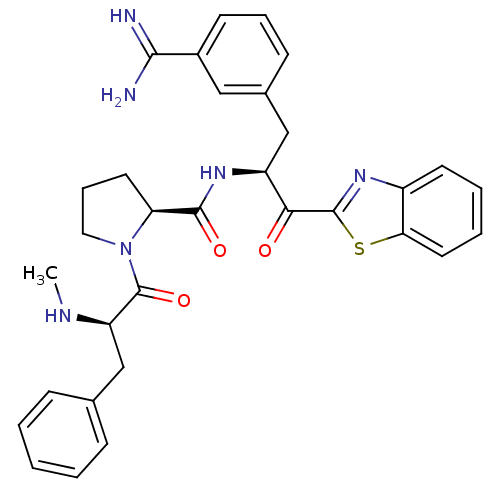
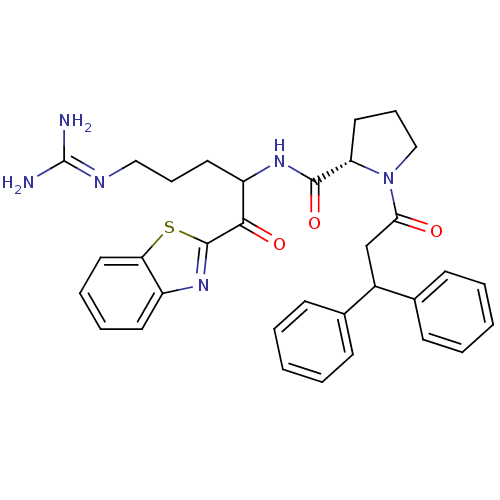
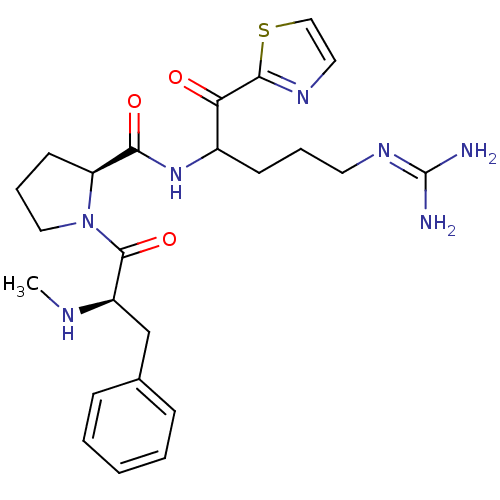
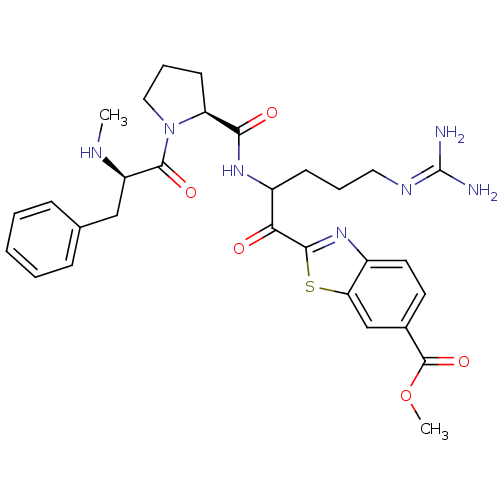
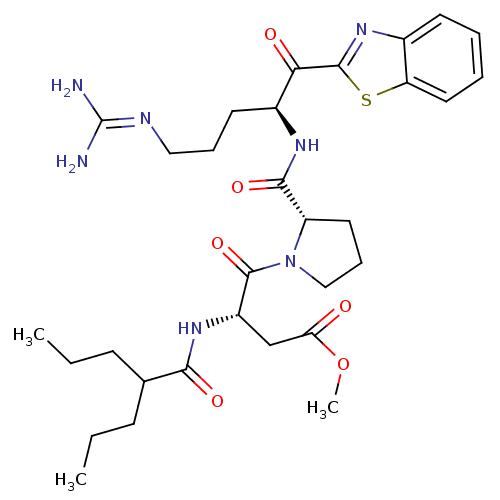
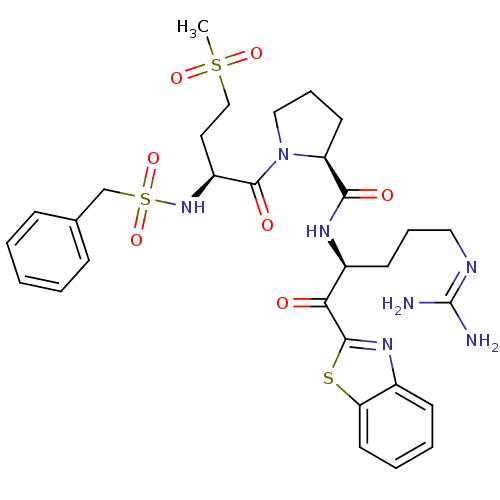
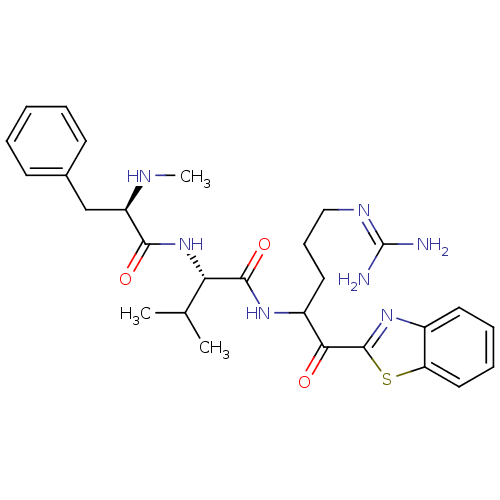
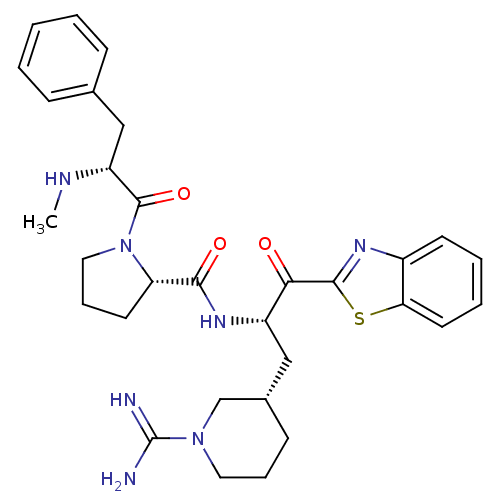
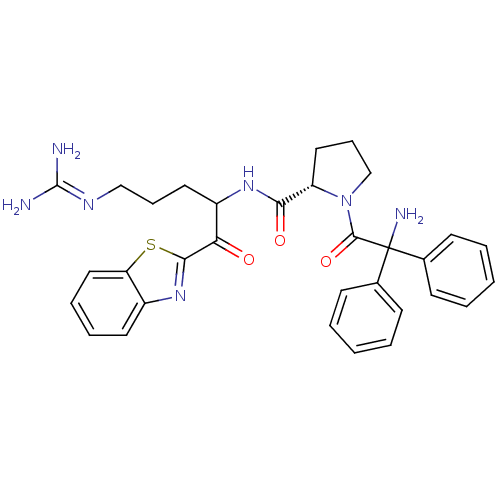
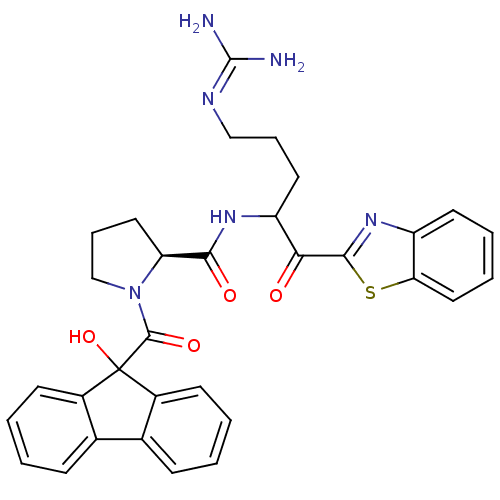
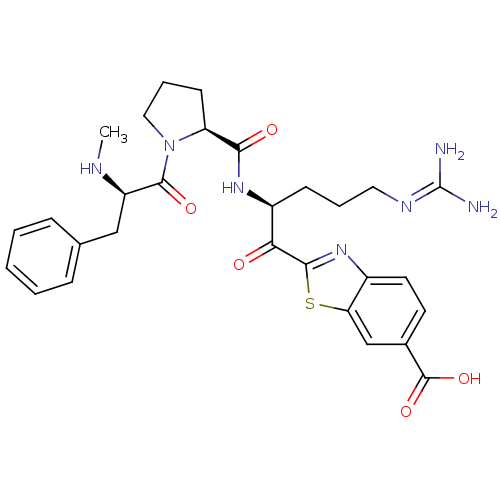
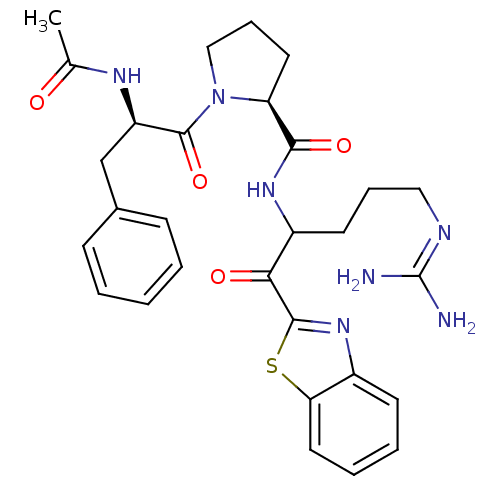
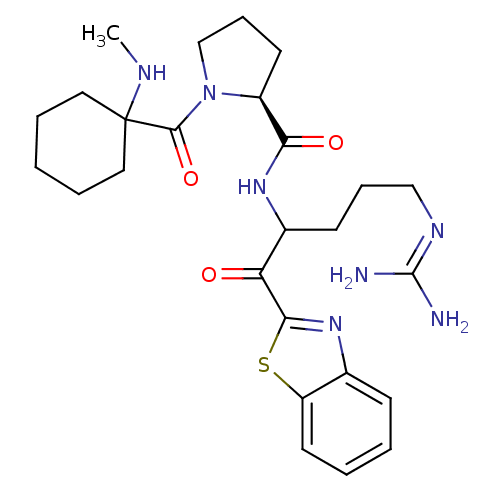
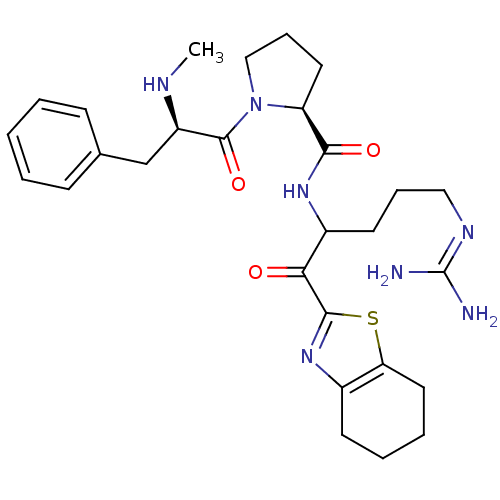
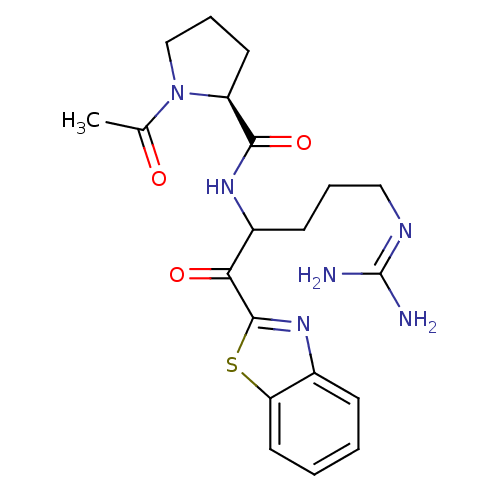
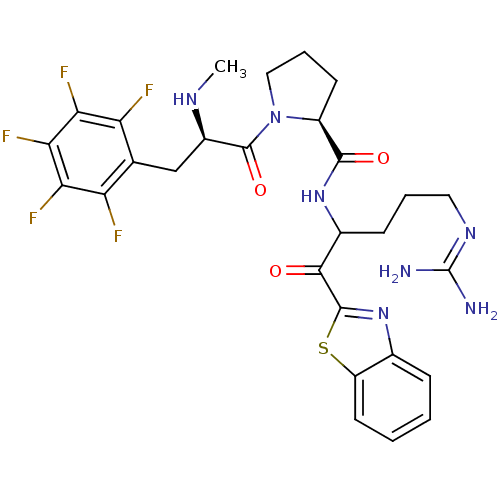
Report error Found 154 Enz. Inhib. hit(s) with all data for entry = 1779
Affinity DataKi: 0.000650nM ΔG°: -72.4kJ/mole IC50: 4.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.00550nM ΔG°: -66.9kJ/mole IC50: 21nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.00700nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.0180nM ΔG°: -63.8kJ/mole IC50: 5.30nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -58.9kJ/mole IC50: 15nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM IC50: 7.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.180nM ΔG°: -57.9kJ/mole IC50: 48nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 3.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.230nM IC50: 3nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.340nM IC50: 38nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.360nM ΔG°: -56.1kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nM IC50: 7nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.460nM ΔG°: -55.4kJ/mole IC50: 34nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.580nM IC50: 60nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.780nM IC50: 110nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -53.2kJ/mole IC50: 11nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nM IC50: 38nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM IC50: 56nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nM IC50: 95nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM IC50: 51nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: 45nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -51.5kJ/mole IC50: 15nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM IC50: 19nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM IC50: 30nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM IC50: 95nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nM IC50: 80nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nM ΔG°: -50.0kJ/mole IC50: 150nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair