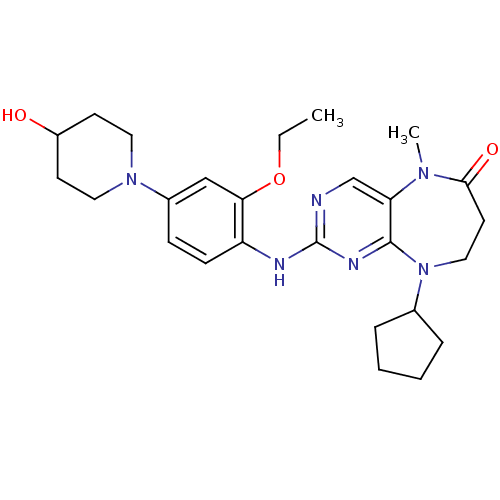
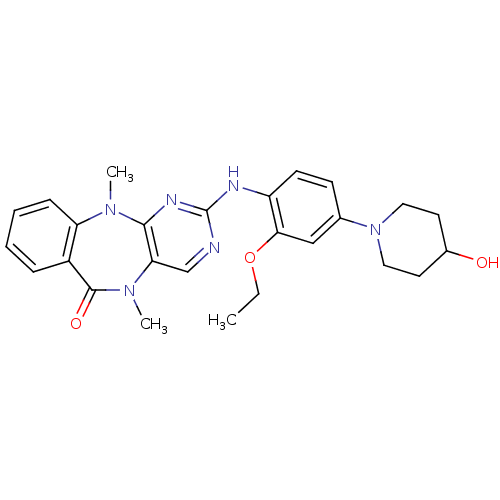
Report error Found 10 Enz. Inhib. hit(s) with all data for entry = 4659
Affinity DataIC50: 8nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 74nM Kd: 9.5nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataKd: 13nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataKd: 15nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 49nM Kd: 23nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 145nM Kd: 26nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 49nM Kd: 40nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataKd: 80nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
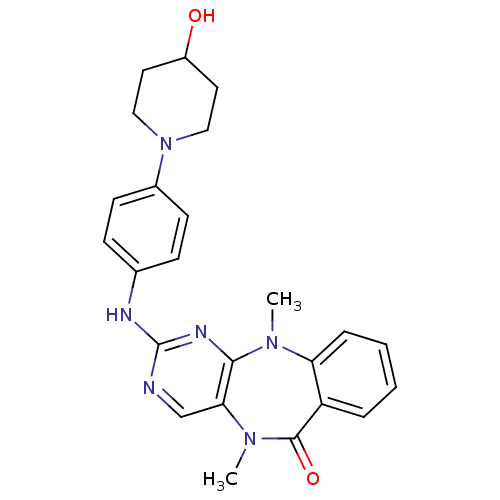
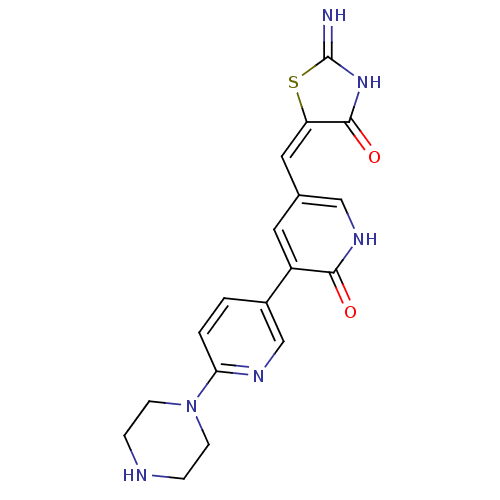
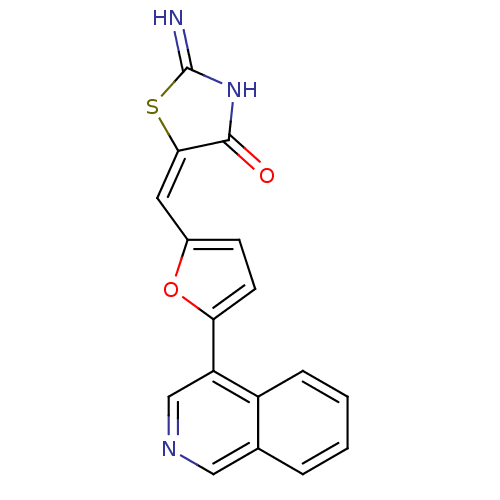
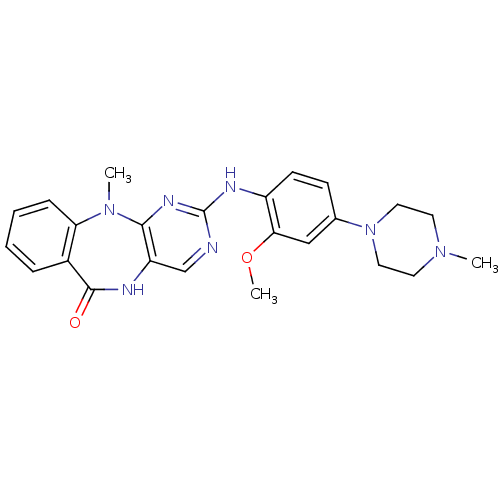
 3D Structure (crystal)
3D Structure (crystal)