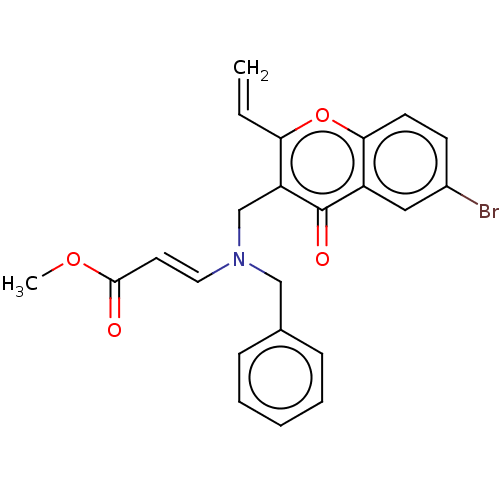
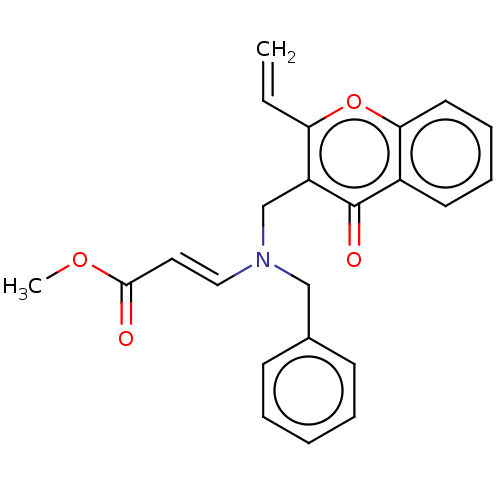
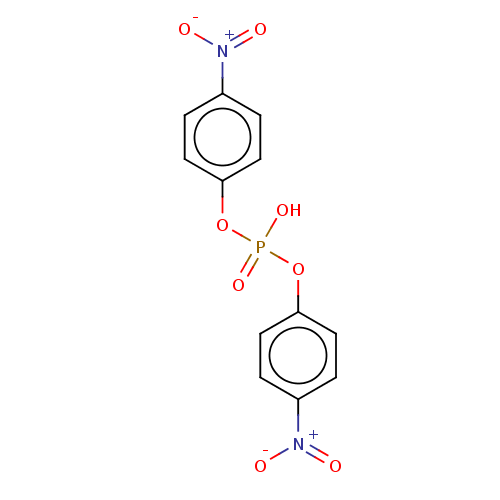
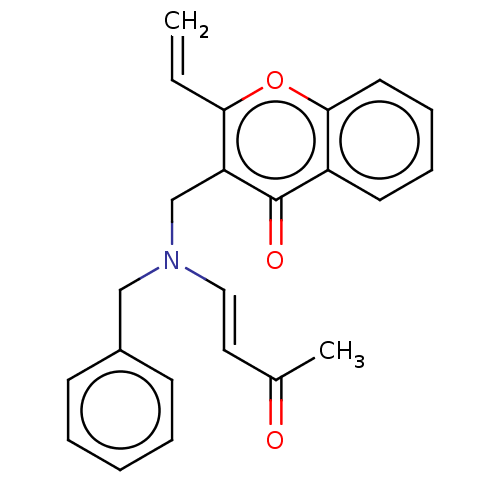
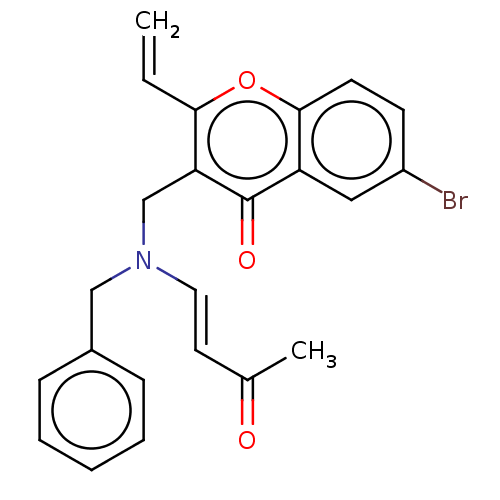
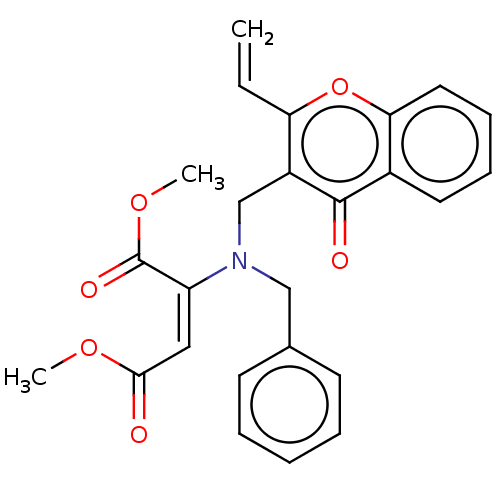
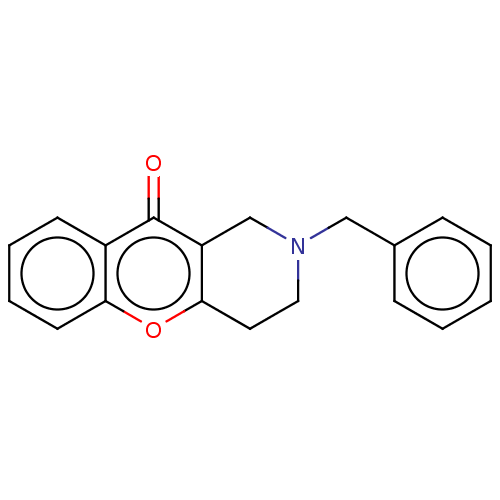
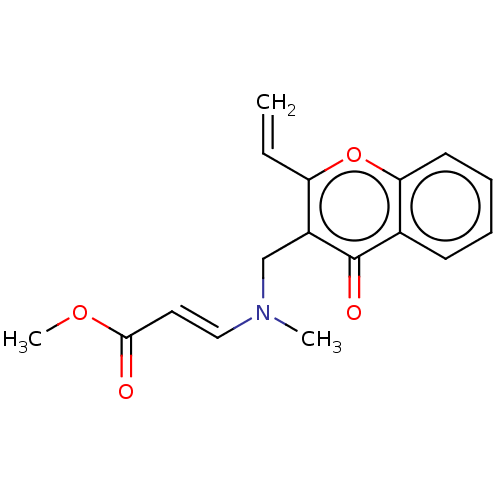
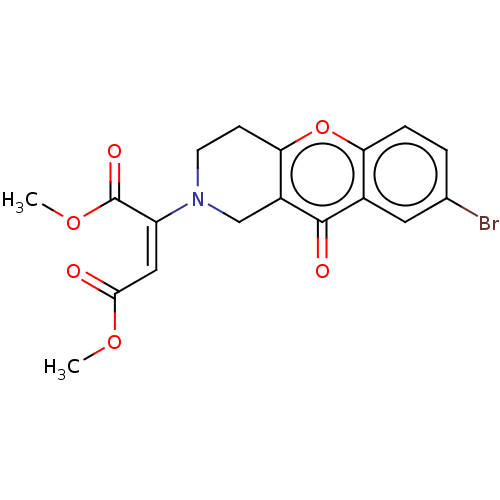
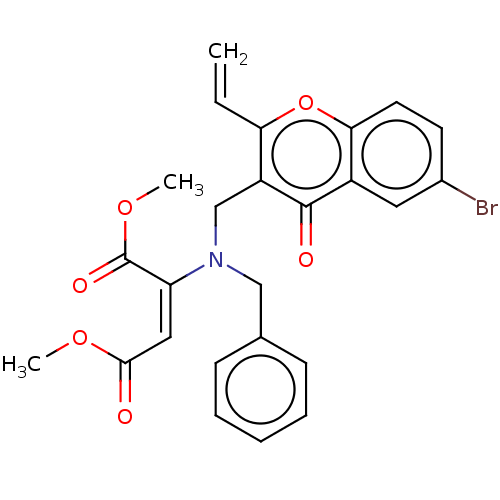
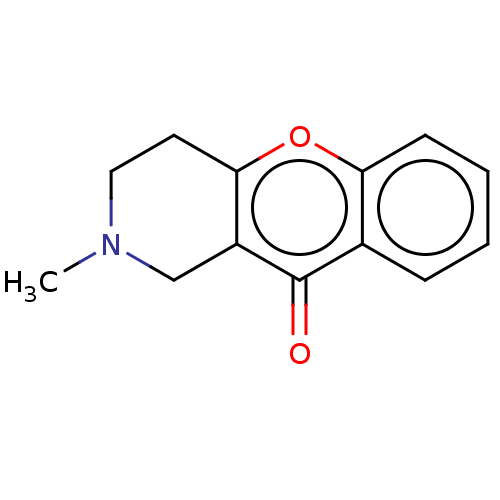
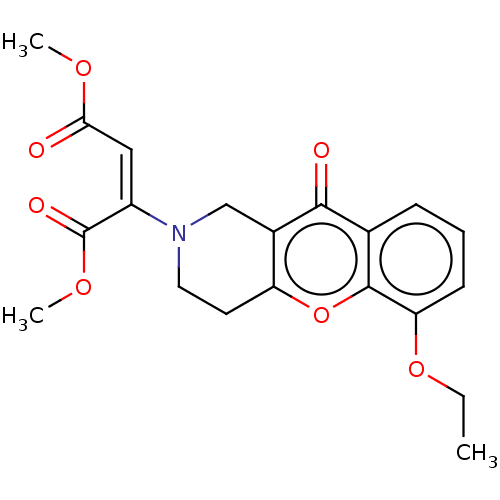
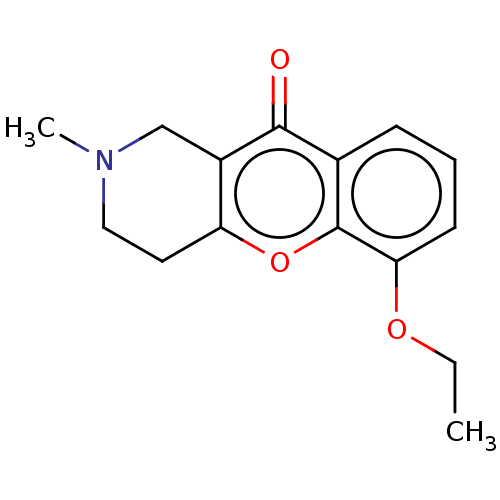
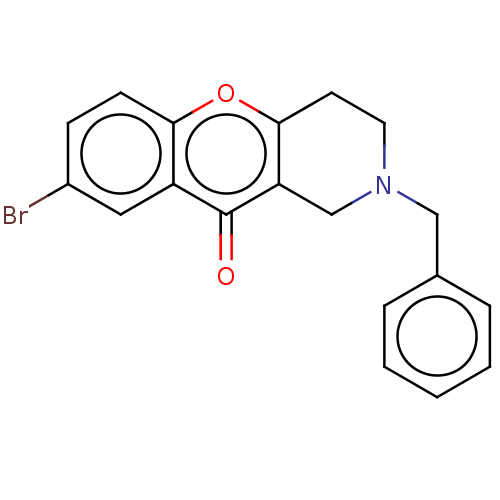
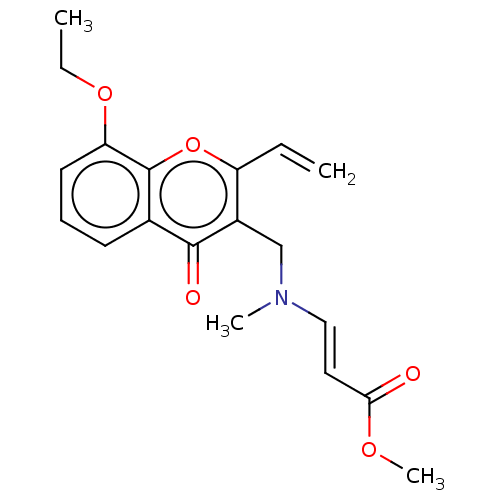
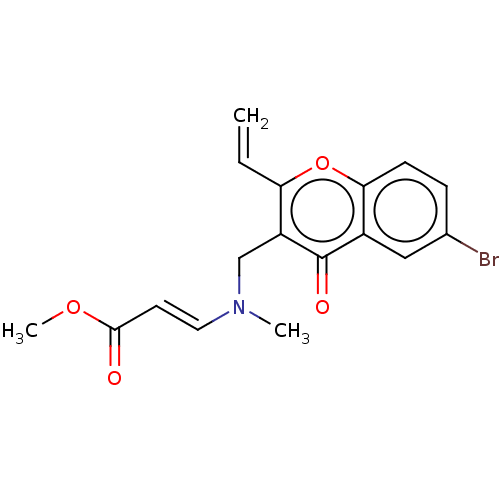
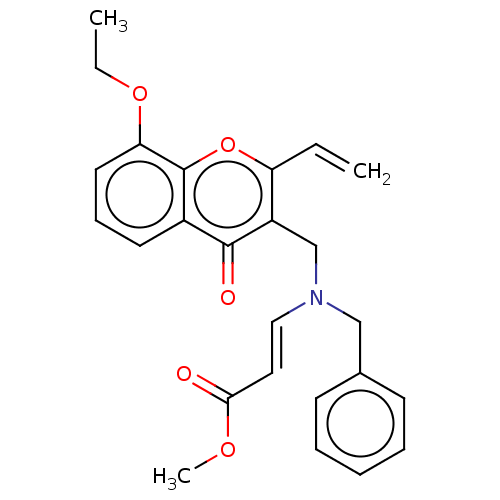
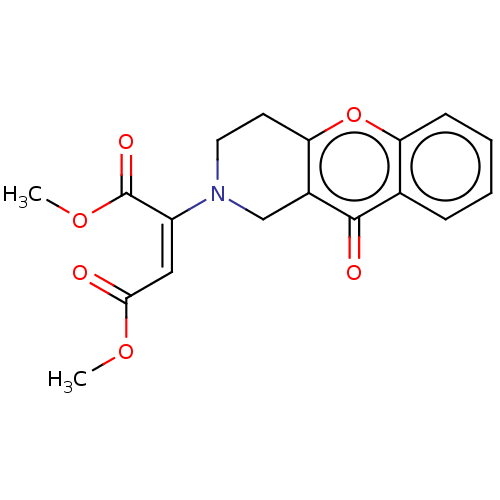
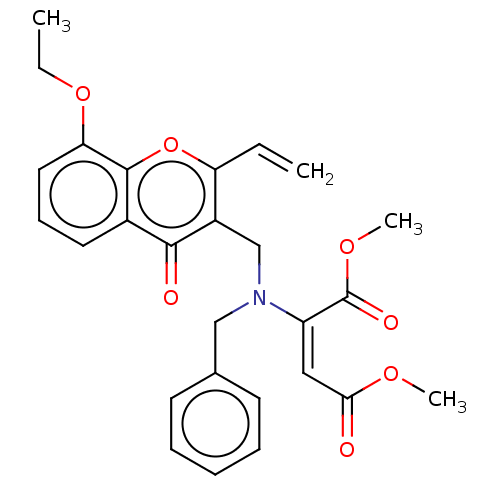
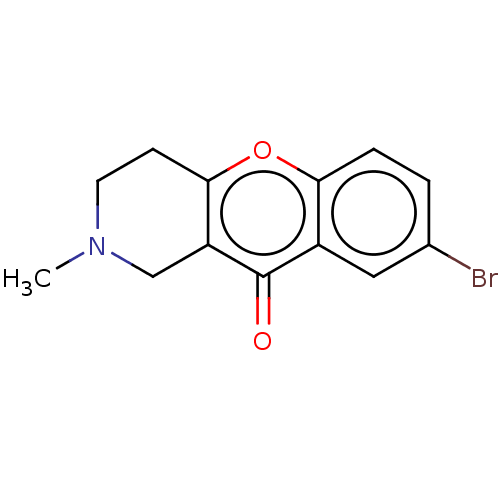
Report error Found 55 Enz. Inhib. hit(s) with all data for entry = 50002846
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
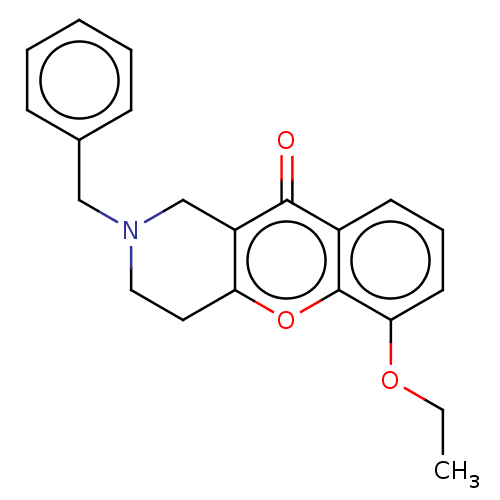
Affinity DataIC50: 29nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.55E+3nMAssay Description:Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.79E+3nMAssay Description:Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 2.12E+3nMAssay Description:Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.27E+3nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.75E+3nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.35E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.38E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Pig)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)