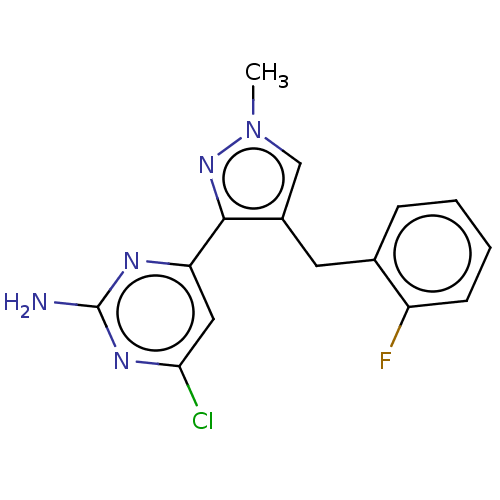
BDBM50577236 CHEMBL4857305::US20240239774, Example 17
SMILES Cn1cc(Cc2ccccc2F)c(n1)-c1cc(Cl)nc(N)n1
InChI Key InChIKey=URARPYRWSZLAND-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50577236
Found 2 hits for monomerid = 50577236
Affinity DataIC50: 120nMAssay Description:Assays for sAC activity using purified protein were performed in 100 ul reactions containing 4 mM MgCl2, 2 mM CaCl2, 1 mM ATP, 40 mM NaHCO3, 50 mM Tr...More data for this Ligand-Target Pair
Affinity DataIC50: 132nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair